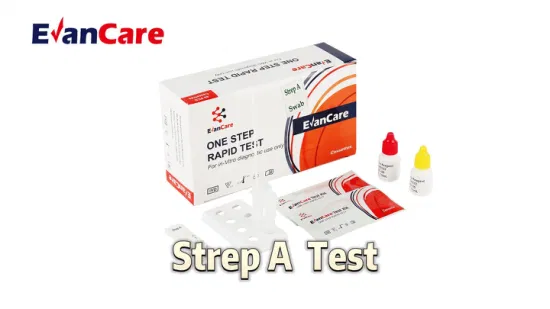
Accurate Antigen Rapid Test Kit Home Use Rapid Strep a Test Cassette with CE
Overview Product Description EVANCARE Strep A Test Diagnostic Kits Strep A Rapid Test Device Swab /Infectious Disease Di
Description
Basic Info.
| Package | Bottle + Carton |
| Material | Medical Grade ABS Plastic |
| Display | Screening |
| Function | Early Detection |
| Method | Colloidal Gold Rapid Test |
| Warranty | 3 Years |
| Certificate | CE, ISO |
| Storage | Room Temperature |
| Delivery Time | 3-5 Days |
| Accuracy | 99.7% |
| Transport Package | Carton |
| Specification | 64*50*44 cm |
| Trademark | evancare or OEM |
| Origin | China |
| HS Code | 3002150090 |
| Production Capacity | 100000PCS/Day |
Product Description
Product Description
EVANCARE Strep A Test Diagnostic Kits Strep A Rapid Test Device Swab /Infectious Disease Diagnostic KitFor professional and in vitro diagnostic use only.
[INTENDED USE] The Strep A Rapid Test Device (Swab) is a rapid chromatographic immunoassay for the qualitative detection of Strep A antigen from throat swab specimens to aid in the diagnosis of Group A Streptococcal infection.
[PRINCIPLE]The Strep A Rapid Test Device (Swab) is a qualitative, lateral flow immunoassay for the detection of Strep A carbohydrate antigen in a throat swab. In this test, antibody specific to Strep A carbohydrate antigen is coated on the test line region of the test. During testing, the extracted throat swab specimen reacts with an antibody to Strep A that is coated onto particles. The mixture migrates up the membrane to react with the antibody to Strep A on the membrane and generate a color line in the test line region. The presence of this color line in the test line region indicates a positive result, while its absence indicates a negative result. To serve as a procedural control, a colored line will always appear in the control line region, indicating that proper volume of specimen has been added and membrane wicking has occurred.
Detailed Photos
Allow the test device, reagents, throat swab specimen, and/or controls to reach room temperature (15-30°C) prior to testing. 1. Remove the test device from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the test is performed immediately after opening the foil pouch. 2. Hold the Reagent A bottle vertically and add 4 full drops (approximately 260μl) of Reagent A to an extraction tube. Reagent A is red in color. Hold the Reagent B bottle vertically and add 4 full drops (approximately 260μl) to the tube. Reagent B is colorless. Mix the solution by gently swirling the extraction tube. The addition of Reagent B to Reagent A changes the color of the solution from red to yellow. 3. Immediately add the throat swab to the extraction tube of yellow solution. Agitate the swab 10 times in the tube. Leave the swab in the tube for 1 minute. Then squeeze the swab in the tube wall as the swab is withdrawn. Discard the swab. 4. Fit the dropper tip on top of the extraction tube. Place the test device on a clean and level surface. Add 3 full drops of solution (approximately 180μl) to the specimen well (S) and then start the timer. 5. Wait for the colored line(s) to appear. Read the result at 5 minutes. Do not read the result after 10 minutes. POSITIVE: Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T). NEGATIVE: Only one colored band appears, in the control region (C). No apparent colored band appears in the test region (T). INVALID: Control band fails to appear. Results from any test which has not produced a control band at the specified read time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor. NOTE: 1. The intensity of color in the test region (T) may vary depending on the concentration of analytes present in the specimen. Therefore, any shade of color in the test region should be considered positive. Note that this is a qualitative test only, and cannot determine the concentration of analytes in the specimen. 2. Insufficient specimen volume, incorrect operating procedure or expired tests are the most likely reasons for control band failure.
Item No. | EVANCARE Strep A Test Diagnostic Kits Strep A Rapid Test Device Swab /Infectious Disease Diagnostic Kit |
Contents | Each bag:1 test cassette and 1 desiccant(cassette) 25 cassettes+25 swab+25 tube +1 work station+ buffer A+Buffer B /box antigen test We can put instruction as your requirement |
Format / Size | Cassette:2.5mm 3.0mm 4.0mm |
Methodology | Sandwich Colloidal Gold |
Assay Type | Qualitative Detection |
Specimen | Antigen (swab), |
Indicator | Strep A Test Cassette |
Storage Temperature | 4-30 degree centigrade |
Expiry date | 3 years |
Certificate | CE,ISO,FREE SALE,FDA... |
Detection Limits | Home or Professional Use |
Accuracy | > 99.99% |
| Reaction Time | 3-5 min |
| Delivery | 5-7 days |
| OEM Service | Available |
| Shipping Method | by air or sea or international express |
Company Profile

Certifications

Product Procedure

Activity Exhibition

FAQ
Prev: Poct Multi
Next: Prostate Specific Antigen Psa Blood Test Individual Package Rapid Test Cassette
Our Contact
Send now






